Free Think – ASAPprime® Software
- Home
- Free Think
- FreeThink – ASAPprime® Software
FreeThink’s ASAPprime® software is the only commercially-available software that enables scientists to quickly and accurately determine substance stability and product shelf-life based on the Accelerated Stability Assessment Program (ASAP). This state-of-the-art statistical software allows for a remarkably accurate prediction of a product’s shelf-life and stability in as little as four weeks.
- ASAPprime® software enables scientists to accurately determine shelf-life and stability of products and substances in as little as four weeks.
- ASAPprime® optimizes packaging selection without screening.
- ASAPprime® module, ASAPdesign™, allows scientists to design exposure conditions (temperature, RH, time) and schedule experiments for shelf-life and stability.
- laboratory work based on the Accelerated Stability Assessment Program (ASAP). ASAPprime® then statistically analyzes the experimental results to determine long-term storage shelf-life.
Accelerated Stability Assessment Program (ASAPprime®)
Alongside the technically based ACE HPLC & UHPLC Product Catalog there are the following Technical brochures available for ACE HPLC & UHPLC columns:
.jpg)
Services

Existing Customers

OFFERING
TRAINING & DOCUMENTATION
Cogent Type-C columns in ANP (Aqueous Normal Phase) mode out perform HILIC columns in the following areas:
- Retain non polar compounds by RP mechanism
- Retain polar compounds by NP mechanism
- BOTH RP and NP mechanisms can operate simultaneously
- Can separate samples with BOTH polar and non polar analytes
- equilibration time for gradient elution is 5 minutes between runs
Glucosamine

LICENSE
- Annual Site License (including all upgrades)
- Two Year License (including all upgrades)
- Three Year License (including all upgrades)
- Additional seats per geographic site:
1.Annual License
2.Two Year License
3.Three Year License
IQ / OQ CERTIFICATION
- IQ/OQ (one computer/one site)
- Additional Computers at same site
SCIENTIFIC CONSULTING SERVICES
- ASAP science, experimental setup, interpretation of data and stability problem resolution
ASAPprime
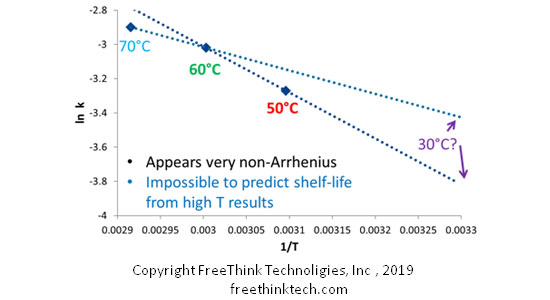
Due to the secondary degradation, the reaction kinetics of the heterogeneous system (multiple mixing), and the participation of reaction inhibitors (such as antioxidants), more than 50% of the drug formulation stability exhibits nonlinear degradation kinetics. That is, the reaction rate changes with conditions and does not follow the classic Arrhenius publicity. As shown in the figure below, if only the temperature factor is considered, the sample is placed at 30 ° C, 60 ° C, 70 ° C for 1 month to measure the impurity mass. Next, the reaction rate (lnk) was plotted against the temperature (1/T). At this time, we found that the reaction rate measured under the three conditions was not in a straight line at all (meaning that the reaction could not be fixed with the parameters of the Aleni The Uz equation describes: The traditional accelerated stability experiment cannot accurately predict the reaction rate under long-term storage conditions.
.jpg)
1. ASAPprime? Solving nonlinear degradation dynamics based on equal transformation principle
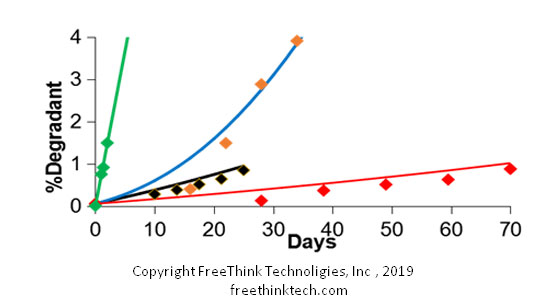
ASAPprime adopts the principle of iso-conversion, that is, the materials under different acceleration conditions are placed to the same degradation limit. Under the same degradation limit, the conversion time shows a good linear relationship with temperature.

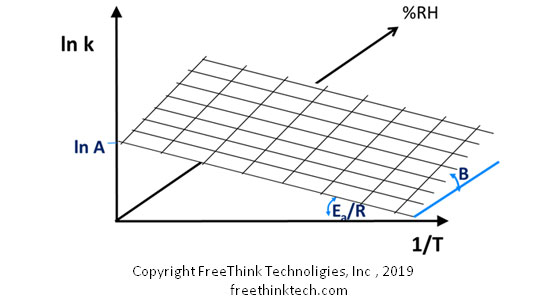
2.ASAPprime? Quantifies the effects of humidity
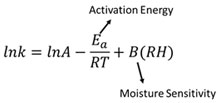
In general, the chemical degradation kinetics of solid materials are affected by both temperature and humidity. ASAPprime improves the classic Arrhenius equation as follows:
3. ASAPprime? experimental design and experimental methods
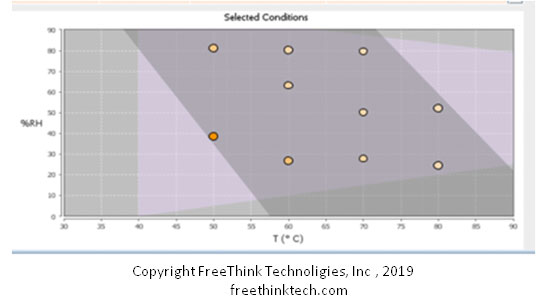
In terms of experimental design, there are three biggest differences between ASAPprime® and ICH stability testing:
- 1. ASAPprime? is a three-dimensional combination of temperature, humidity and time.
- 2. ASAPprime? sample is placed in the opening to accurately control the temperature and humidity of the sample (and real-time recording)
- 3. ASAPprime? is aimed at equal conversion, which is aimed at the similar degree of degradation of each sample.
- 4.ASAPprime? Experimental design optimized in statistics (maximum degree of decoupling temperature and humidity synergy)
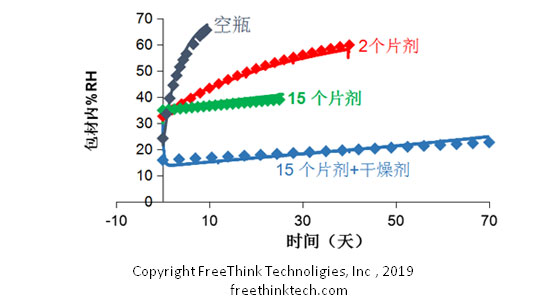
4. ASAPprime? Treatment of water adsorption characteristics and packaging materials
Through the above experimental design, we can know the inherent degradation kinetics of the product, that is, how the shelf life changes with environmental influences. During long-term stability placement, the temperature remains constant and the relative humidity of the product is constantly changing. ASAPprime has a complete methodology to accurately predict the long-term trends of relative humidity in the package over time based on the isothermal adsorption characteristics of the sample and the ability to maintain water resistance. Combined with the intrinsic degradation kinetics of the product, the shelf life of the product corresponding to different packaging materials and storage environment can be accurately predicted.